| 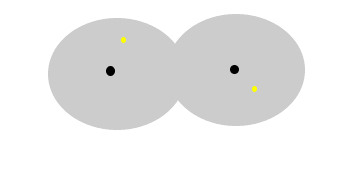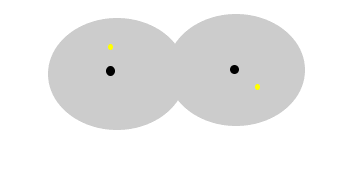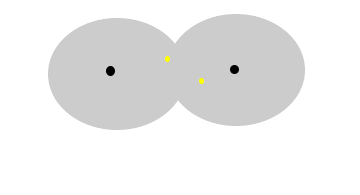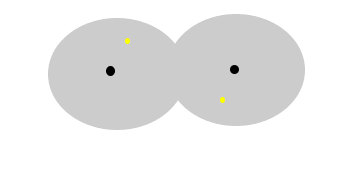
The animation
above show the formation of instantaneous dipoles in a hydrogen molecule
due to the random motion of electrons within the molecule. Notice how
temporary these charges are. |
When two identical
atoms (with the same electronegativity) combine to form a covalent bond
the electrons are shared evenly. Such a covalent bond is called a pure
covalent bond. However, even a pure covalent bond can still result in
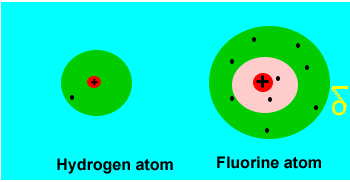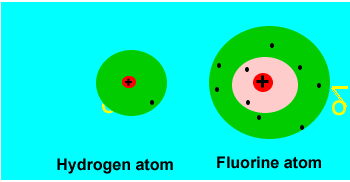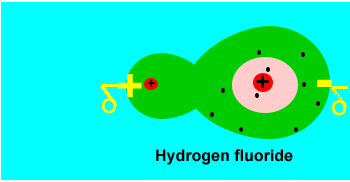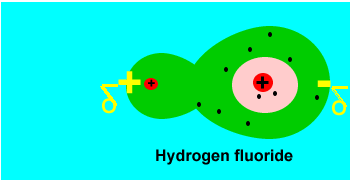
small charges forming on the molecule. Take two hydrogen atoms bonding to
form a molecule. The electrons that are shared move randomly between the
two atoms under the influence of electrostatic forces. At some point in
time, for a small fraction of a millisecond (in other words a very small
amount of time indeed) the two electrons may find themselves on one side
of the molecule. This results in brief positive and negative dipoles forming
on the molecule. Such charges are temporary and disappear as soon as they
form. These charges are given the name instantaneous
dipoles. As you can well imagine the more electrons you have in
a molecule moving randomly the more chance that these instantaneous dipoles
will form more often. This is exactly what happens, in that we see more
frequent instantaneous dipoles forming in bigger molecules than smaller
ones. These instantaneous dipoles create very weak forces of attraction
between molecules called dispersion forces.
|