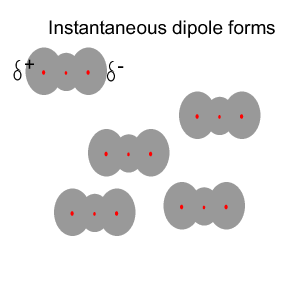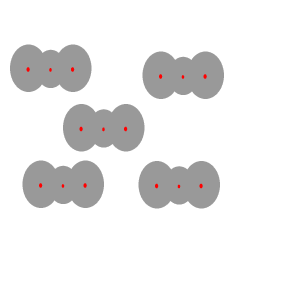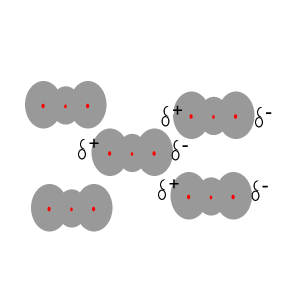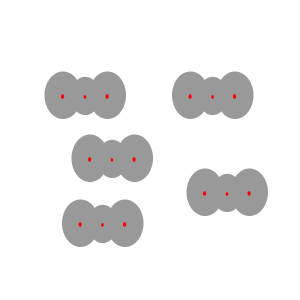
Instantaneous
dipoles and dispersion forces.
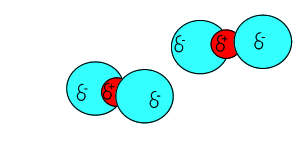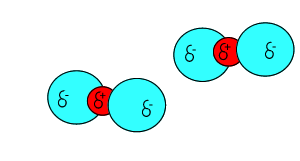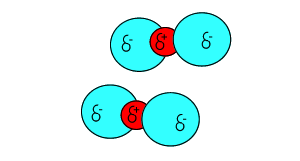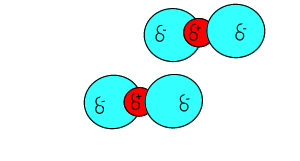
Click to see the polar covalent nature of the intramolecular bonding.
What is this force of attraction and how does it come about?
The intermolecular forces that act between the CO2 molecules are known as dispersion forces. These forces of attraction come about due to the formation of instantaneous dipoles. Instantaneous dipoles are not permanent and as the name implies, they exist for only an instant and their formation is purely random. Instantaneous dipoles come about due to the random movement of electrons within the molecule. Click to see an animation.