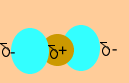


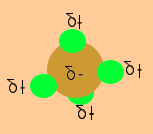
The methane molecule on the right is symmetrical. The negative charge on the carbon is completely surrounded by the positive charges on the hydrogen atoms.The negative carbon is not exposed to the other molecules.
Click to see an animation of the process
Since symmetrical
molecules either have no charge(O2) or have a similar charge
on the exterior (CH4). In either case no attraction or repulsion
occurs and it is hard to imagine that a force of attraction actually
exists. The fact that we can form solid carbon dioxide(dry ice) and
liquid methane is evidence of an intermolecular force of attraction
acting between the molecules of each compound.
Such intermolecular forces of attraction are known as dispersion
forces or van der waal's forces. Click
for a more detailed explanation of how these forces come about.