Changing the properties of metals
|
| Metals can be modified in a number of ways. Alloys are metals with other element mixed in. We discussed alloys previously. The properties of iron can be changed by work hardening and heat treatment. |
| Many metals are modified while in the molten state. Iron for example, is formed into steel y dissolving carbon in the molten iron. |
 |
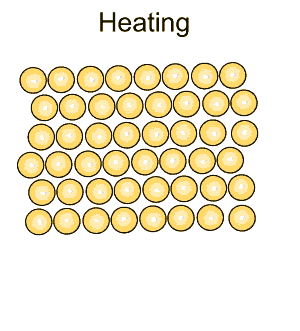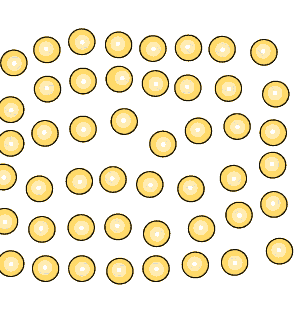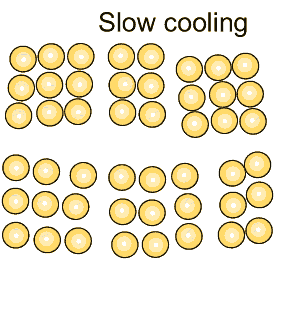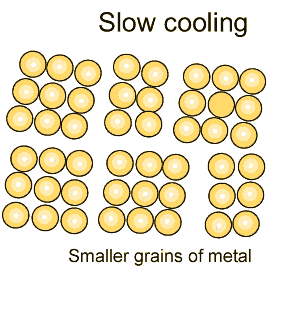
| The rate at which a metal is cooled has a significant impact on its properties. A metal is made of many small grains packed tightly together. The rate of cooling of hot metals as well hammering (work hardening) changes the size of the grains and makes the metal harder and more brittle. |
 |
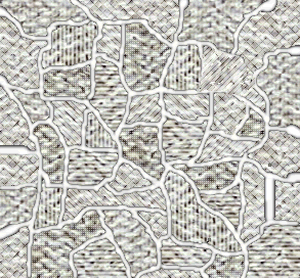
Pure iron is a soft metal that is useless for the construction industry. When iron is heated to just over 900oC and then cooled, groups of atoms combine together to form grains, as shown on the right. Grains are separated by boundaries called dendrites. |
|
Grain size greatly affects the toughness of steel. The smaller the grain size, the more ductile the steel.
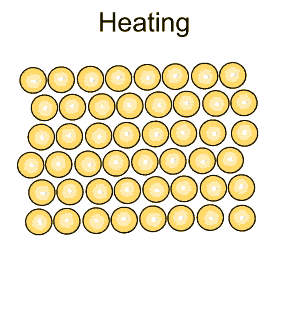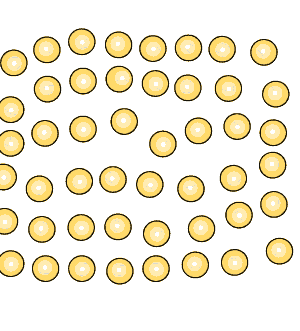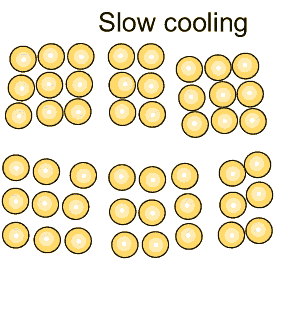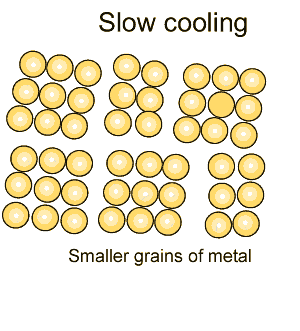
When steel is cooled slowly large grains form. When steel is cooled quickly smaller grain size is the result which makes the steel more ductile.
|
|
The more grain boundaries that exist, the more difficult it is for groups of atoms to move, and as a result, the steel is harder and stronger.
Coarse grained steel contains fewer grains and grain boundaries than fine grained steel with many more grains and boundaries. Because of this, fine-grained steel has the potential to be stronger and more ductile than coarse grained steel. |
|
|