Chemical reactions
between molecules
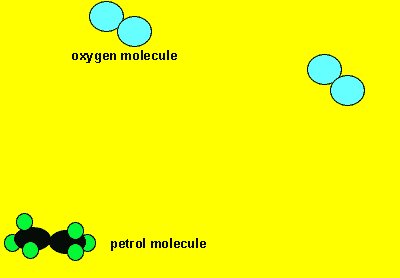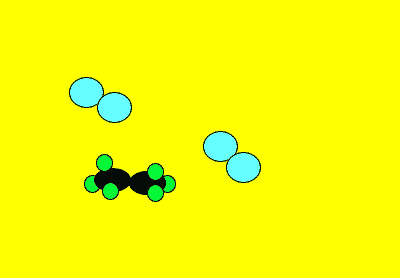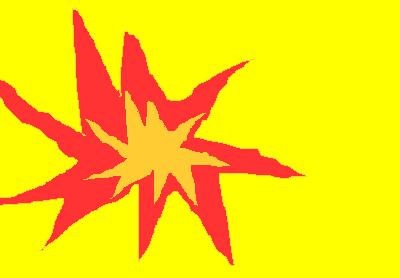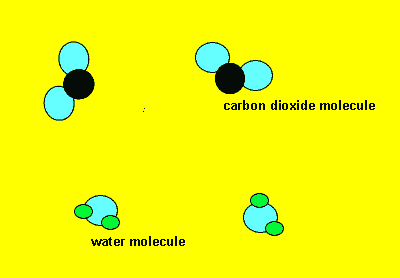
Neutral molecules can also react to form new products. Sometimes these reactions produce a great deal of energy as well as the formation of new products. Click to see a 120kb video.
Such reactions rely on the force with which the molecules collide in order to break apart the molecules into their component atoms. Once parted the atoms quickly recombine to form more stable products. For some reactions there is enough energy in the solution they are in to cause a reaction to occur. For example when we add vinegar to bicarb soda(baking powder) carbon dioxide is immediately released. Click to see the video. Obviously molecules from the vinegar and the baking powder are breaking apart releasing atoms that recombine to form carbon dioxide gas.
Sometimes in order for a reaction to proceed we must supply a little energy. You would have noticed this when you burn petrol or paper. A flame must be supplied to start the burning. Once on fire the paper or petrol releases its own energy which keeps the reaction going. The initial energy that must be supplied to get the molecules colliding is known as the activation energy.