Thermochemical equations
Exercises
C8H18(g) + 12.5O2(g) => 8CO2(g)+ 9H2O(g) ΔH = -5054kj/mol
1) Calculate the
energy released when 34.5 litres of octane gas react with oxygen at STP
according to the reaction above.
C = 12, H =1, O =16.
Solution
2) Calculate the amount of energy released when 40 grams of glucose burn in oxygen according to the equation below?
C6H12O6(aq) + 6O2(g) => 6CO2(g)+ 6H2O(l) ΔH = -2803kj/mol
Solution
3) Calculate the amount of energy absorbed when 40 grams of carbon dioxide are used in photosynthesis to produce glucose according to the equation below.
6CO2(g)+ 6H2O(l) => C6H12O6(aq) + 6O2(g) ΔH = +2803kj/mol
Solution
4) Calculate the mass of octane needed to produce 760kj of energy when it reacts with oxygen according to the equation below.
2C8H18(g) + 25O2(g) => 16CO2(g)+ 18H2O(g) ΔH = -10108kj/mol
Solution
5) Calculate the mass of glucose needed by a plant in order to produce 340kj of energy during respiration.
C6H12O6(aq) + 6O2(g) => 6CO2(g)+ 6H2O(l) ΔH = -2803kj/mol
Solution
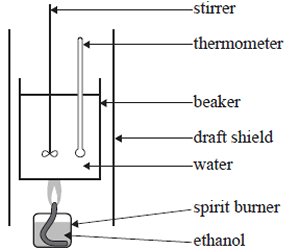
6) A student experimentally determined the molar enthalpy of combustion(ΔHc) of ethanol, molar mass of 46.0 g/mol, using the equipment shown in the simplified diagram on the right..
The student made the following experimental measurements
Mass of water in beaker = 105g
Amount of ethanol combusted = 0.920g
Temperature rise of the water = 39.0 oC
If the specific heat capacity of water is 4.184 J/g/oC find the molar enthalpy of combustion of ethanol, calculated from the student's results.
Solution
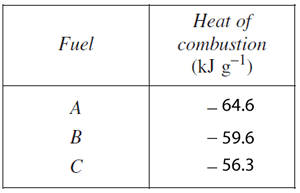
7) A student measured the heat of combustion of three different fuels using similar equipment as that shown in 6) above. The results are shown in the table on the right. The published value for the heat of combustion of propane is 2217 kJ mol−1.
Which fuel from the table is likely to be propane? Justify your answer .
Solution
8) Instant cold packs are often used by athletes to reduce swelling just after injury. Such cold packs often contain ammonium nitrate. When needed, the ammonium nitrate is mixed with water and the following reaction occurs dropping the temperature.
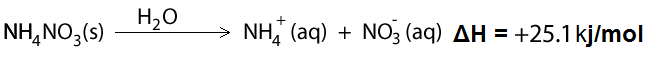
The above process occurs in two stages. Firstly, energy is required to break the ionic bonds which hold the ions together in the solid state. This energy is taken from the surrounding water. Finally, energy is released when the ions and water molecules form ion-dipole bonds to produce stable complexes.
1)
What can you say about the amount of energy required to break the bonds as compared to the amount of energy given out when ion-dipole bonds formed?
2) A cold pack contains 50.0 g of solid ammonium nitrate that is mixed with water when activated. What is the amount of energy that is released or absorbed by the reaction?

Click for more exercises from past VCE examinations.
2011 VCE 2010 VCE 2009 VCE 2008 VCE 2007 VCE 2006 VCE 2005 VCE