Proteins are large molecules made of smaller molecules known as amino acids. When you look in the mirror you see protein and the effects of protein molecules within the body. Proteins are the most complex organic molecules in Nature. There are many thousands of different proteins, each with its own unique purpose.
Some examples of the many varied tasks that proteins perform in our bodies are listed below.
Structural proteins -
form the skin, exoskeletons, tendons, cartilage, feathers, muscle.
Enzymes - assist in digestion and metabolism.
Hormones - regulate our body
Immune system - forms antibodies.
Animal toxins -most poisons are proteins.
Proteins can be described as polymers formed from the linking of many monomers known as amino acids. Amino acids have two common functional groups a carboxyl and an amino group. All amino acids share the same basic structure with the only difference being the "R" group.
| "R" |
Name |
| H |
Glycine |
| CH3 |
Alanine |
| HOCH2 |
Serine |
Some amino acids can be synthesized by the body but nine, out of twenty amino acids, are known as essential amino acids because they have to be part of the animal's diet. Essential amino acids can not be synthesized.
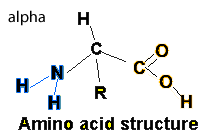
Having carboxyl and amino functional groups make the amino acids very polar and hence soluble in water.
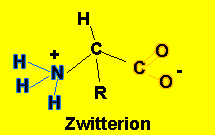
At a certain pH amino acids in solutions have the form shown on the left known as a zwitterion. The acidic carboxyl group has lost a proton which is taken up by the basic amino group. The pH at which an amino acid exists as a zwitterion depends on the R group attached to the amino acid. This pH is known as the isoelectric point (pI)
If the pH of the solution, the amino acid is placed in, is equal to its pI the amino acid will have no net charge.
NOTE - at the isoelectric point it is only the carboxyl group on carbon 1 and the amino group (NH2) on carbon number 2 that become charged.
.
If the pH of the solution the amino acid is placed in is greater than its pI, the amino acid will have a net negative charge. This is due to the fact that the COOH group acts to give up a proton in the relatively basic solution to form an negative charged ion (COO- ).
The ability of amino acids to react with both acids and bases make them useful as buffers.
Below are the pI values of glutamic acid, glycine and lysine.
| Glutamic acid | (R = -CH2CH2CO2-): | pI = 3.1 | ||
| Glycine | (R = -H): | pI = 6.1 | ||
| Lysine | (R = -CH2CH2CH2CH2NH3+): | pI = 10.0 |
Buffers maintain the pH levels within narrow limits. Their buffering action or ability to mop up H+ and OH-, make them very important chemicals to cells as biochemical processes only occur within a very narrow pH range.
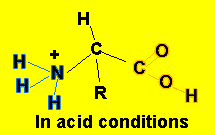
- In acid conditions, the following equilibrium takes place
H2N-CHR-COOH(aq)
+ H3O+(aq) ![]() H3N+-CHR-COOH(aq)
+ H2O(l)
H3N+-CHR-COOH(aq)
+ H2O(l)
This equilibrium mops up H3O+ ions.
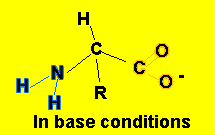
- alkaline conditions, the following equilibrium takes place
H2N-CHR-COOH(aq)
+ OH-(aq)![]() H2N-CHR-COO-(aq)
+ H2O(l)
H2N-CHR-COO-(aq)
+ H2O(l)
This equilibrium mops up OH- ions.
Because of the two equilibria shown above, amino acids can act to control the pH of a solution