The gas laws and cloud formation

Pour some warm (not hot) water to a depth of 2 cm in a 2 litre plastic bottle. Wait a minute for the air inside the bottle to become saturated with water vapour. Light a match, put it out, and place the smoking, extinguished match inside the bottle. Cap the bottle and squeeze tightly. When you release the pressure on the bottle you will notice a cloud forming inside the bottle. When you apply pressure the cloud disappears.
When we increase the pressure of the gas, by compressing it, the temperature increases. When we release the bottle and the gas expands, under low pressure, the temperature falls and the water vapour condenses. Water vapour will condense on surfaces such as the container wall. The smoke provides fine particles in the air inside the bottle which the water molecules condense on to form a cloud.
Now this is a sealed container so the amount of gas is constant.
Remember the combined gas law.
Warm air rises and expands as the pressure drops. An increase in volume cools the gas down. The image on the right shows the temperature of the air every 1,000 metres and the temperature of a rising body of air.
As the body of air rises it cools down and gives off energy, heat, to the surrounding air in the upper atmosphere. Some of this heat is radiated out into space and helps maintain Earth's temperature balance.
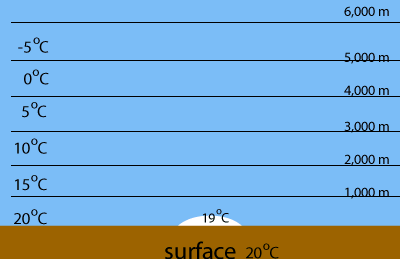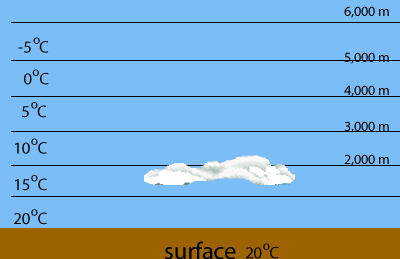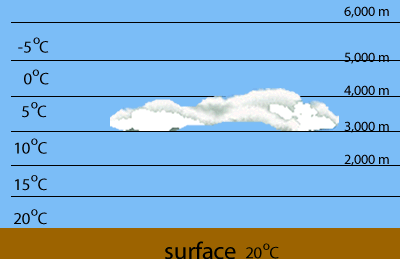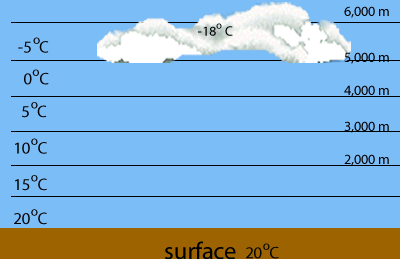
Evaporation of water is highest at the warmest parts of the Earth, such as the tropics. When warm, water saturated air is forced to rise it cools and condenses the water the water vapour it contains.
This warm air can be forced to rise by mountain ranges or when it meets a cold body of air.
Different types of clouds can form two are listed below.
High clouds known as cirrus.
Mid-level clouds known as lenticular altocumuli
Vertical cloud known as cumulonimbus

A mol (6 X 1023 molecules) of water has a density, at S.T.P (0 oC and 1 atm pressure) of 18.0 g/mL. Dry air has a density, at S.T.P. of
28.6 g/mL. Knowing this suggest why clouds float in the air.
Moist air from the tropics meets a body of air, at the same temperature, from the desert. Suggest, with reasons, how the colliding bodies of air will behave?
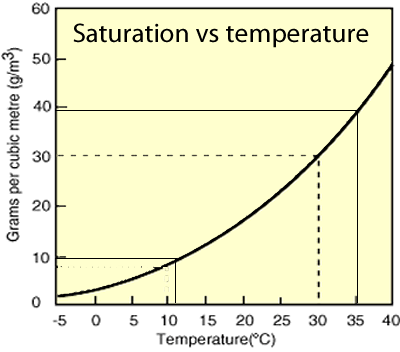
The graph on the right shows the maximum mass of water that a cubic metre of air can hold at a given temperature.
What is the mass of water contained in 5 cubic metres of air at 35 oC ?
What mass of rain would fall if ten cubic metres of air at 30 oC was suddenly cooled to 11 oC ?
Why does a warm body of air rise as it meets an oncoming cold body of air?
Why does the bottle go clear when the pressure is released?
A body of air heats up over a warm ocean and rises to high altitude. What happens to the warm body of air and the surrounding air when it rises to high altitude?