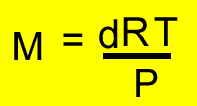 or
or 
(1)
Find the formula weight of an unknown gas, if 0.98 grams of it occupies a volume of 440ml at 23oC and 749mmHg pressure.
Clues
Solution
Step
1- Find the pressure the gas exerts in kPa.
Step
2- Convert the temperature
from Celsius to Kelvin.
Step
3- Convert the volume from
millilitres to litres.
Step
4- Solve for the molar mass
Click on each step
for extra assistance
Click to hide
The pressure of the gas is 749mmHg.
We must convert the pressure from mmHg to kilopascals.
760mmHg = 101.3kPa
So
(740/760) X 101.3 = 98.63kPa
Click to hide
23 degrees Celsius is (273 + 23) = 296o K
Click to hide
The volume needs to be converted
to litres.
To convert millilitres into litres we divide by 1000
440ml = 0.44litres
Click to hide
Place the values of each variable in the formula and solve for the formula weight.
M = (0.98 X 8.31 X 296) / (98.63 X 0.44) = 55.55 atomic mass units
Click to hide
|
The
ideal gas equation
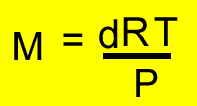 or or  (1) |
|
Find the formula weight of an unknown gas, if 0.98 grams of it occupies a volume of 440ml at 23oC and 749mmHg pressure.
|
Back to the ideal gas equation