Evidence for Human carbon emissions.
Isotopic fingerprint.
First lets have a look at what is an isotope. An isotope is an atom of the same element but with a different number of neutrons in its nucleus. For example carbon has three different isotopes of mass 12, 13 and 14. Only the carbon-12 and carbon-13 isotopes are stable and found in nature. Carbon-12 is the most abundant and makes up 98.93% of all carbon on Earth, while the carbon-13 makes up only 1.07%.
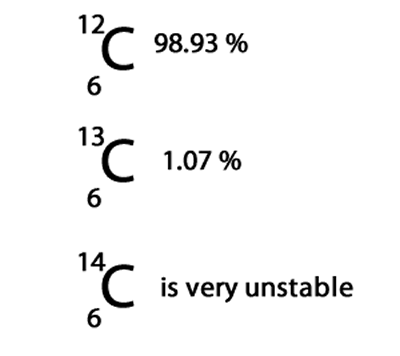
Now we have to ask. Why is it that plant and animal matter contains a higher ratio of 12C to 13C than that found in the atmosphere?
Well, the 12C isotopes have a greater speed than the 13C isotopes at any given temperature. Because they are faster, the 12C carbon atoms get into the plants, through tiny holes on the surface of leaves, more readily than the slower 13C. The space inside the leaf has now a slightly greater proportion of 12C atoms than the atmosphere.
Not only has the space inside the leaf a greater proportion of 12C atoms, but the carbon dioxide molecules with the lighter 12C isotope travel faster than the molecules with the 13C isotope and so get to collide with other reactant molecules more frequently. Due to this higher frequency of collisions the lighter molecules are preferentially used in the formation of plant matter than the heavier molecules .
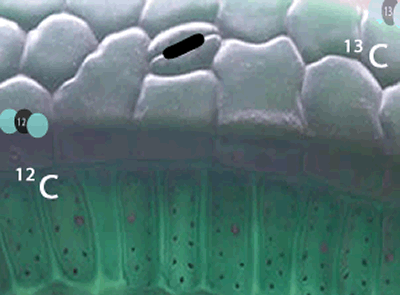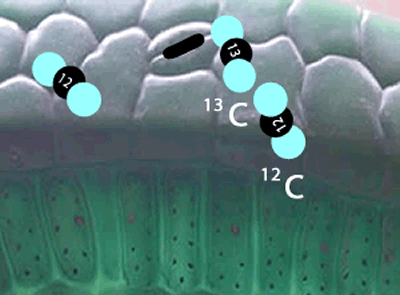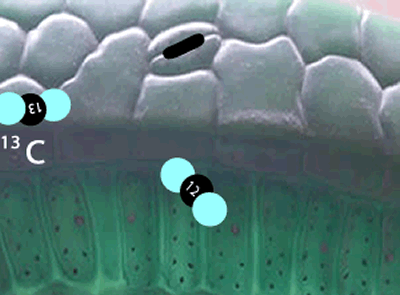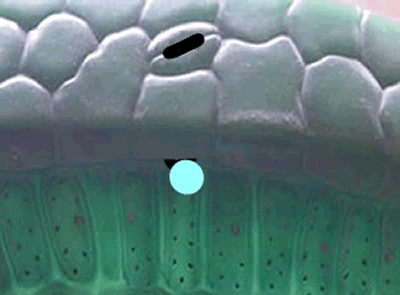
Enzymes that are involved in photosynthesis prefer the carbon dioxide molecules with the 12C isotope over the carbon dioxide molecule with the 13C isotope. There is more vibrational energy in the bonds of the lighter carbon dioxide molecule and hence it is easily broken when compared to the heavier molecule containing the 13C isotope.
In short, the heavier the atom involved in the bond, the more energy is needed to break this bond. So a heavier isotope will form a stronger bond compared to a lighter isotope. More energy is therefore needed to break the stronger bond and this results in a slower rate of reaction when the heavier isotope (13C) is used in photosynthesis when compared to the 12C isotope.
What this means is that a greater proportion of 12C atoms are used in the formation of sugar during photosynthesis than the heavier 13C isotope.
So we see that plant and animal matter has a greater proportion of 12C isotopes than the atmosphere.
With very sensitive instruments we can now analyse samples of the atmosphere. By taking samples of ice cores that have trapped bubbles of air over hundreds of thousands of years we can study the composition of ancient air bubbles. What the data reveals is that the proportion of 12C is increasing in the atmosphere since the industrial revolution.
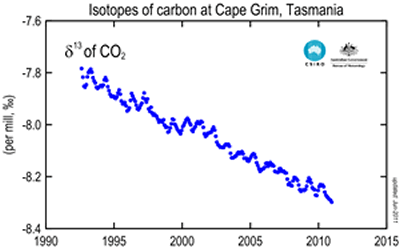
The image on the right shows the change in the ratio of 13C to 12C . This ratio is written as δ13C and is measured in parts per thousand. The graph on the right clearly shows the decline in the ratio
13C : 12C, even as the atmospheric concentration of carbon dioxide is increasing over the same period of time.
Offer an explanation.
Click for a larger image