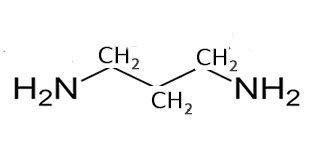
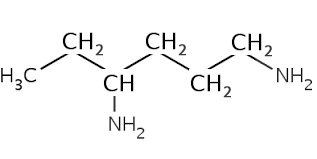
Compounds in which the principal functional group is the amino functional group (–NH2 ) are called amines and so ‘amine’ should appear in the systematic name.
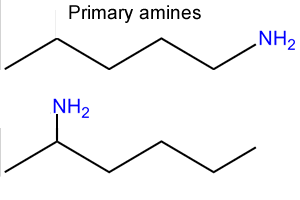
Step 1 We identify the longest carbon chain in the molecule. that includes the amino group (-NH2). Number the carbons so that the amino group is on the lowest number carbon.
Step 2 Replace the "e" of the alkyl group with the longest chain is changed to "amine".
Step 3 The chain is numbered to locate the amino group and substituents. |
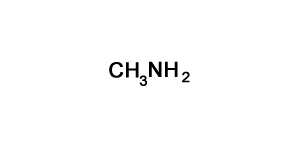
Solution
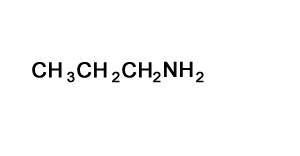
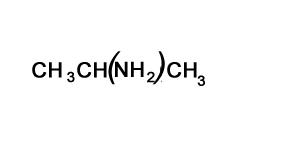
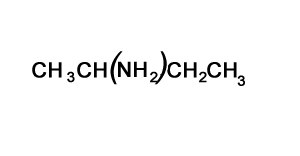
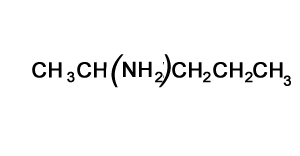
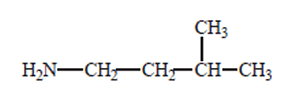
Solution
Past exam question
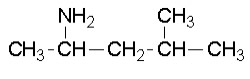
Solution
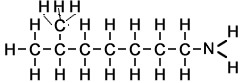
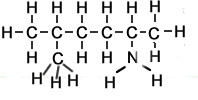
What about if an amine has two NH2 groups? Will we count the longest carbon chain that exists between the two groups, keep the end "e", number the carbons so they have the lowest numbers and use the suffix diamine. Lets see some examples