Electrolysis
in solutions exercises solution 1
Students should be aware that oxidation (the loss of electrons) occurs at the anode and reduction (the gain of electrons) occurs at the cathode.
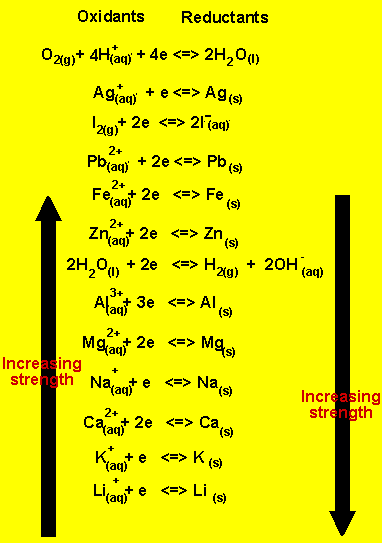
1) An aqueous solution of copper sulfate is electrolysed. Use the reactivity series on the right.
a)What are the products formed
at the anode and at the cathode?
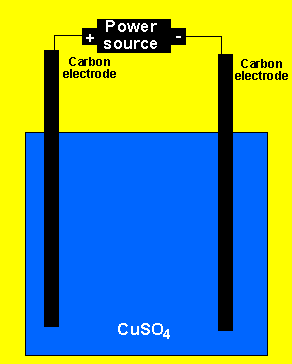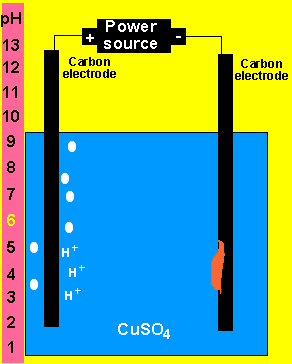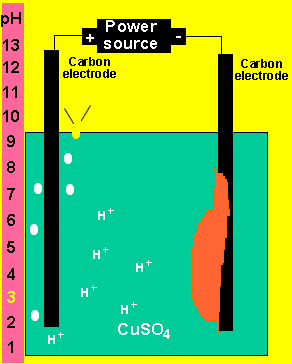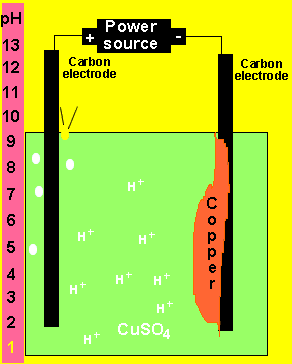
At the anode (+) oxygen and hydrogen ions are formed according
to the equation below.
2H2O(l) => O2(g) + 4H+(aq) + 4e
At the cathode(-) copper metal is deposited according
to the equation below.
Cu2+(aq)+ 2e => Cu(s)
b) What happens to the pH of the solution?
As hydrogen ions are formed the pH will slowly decrease.