Solution/Concentration
Soluble compounds dissolve in water to form
a solution. A solution is made up of two parts, the solvent and the solute.
Concentration of a solution describes the relative proportion of solute and solvent. When the ratios of solute to solvent is very high the solution is said to be concentrated. If the ratio is very low we use the term dilute to describe the solution.
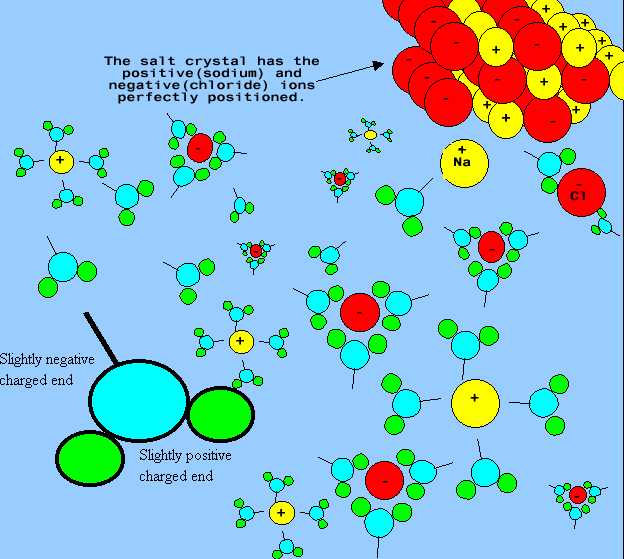
The solvent is the substance that the solute is dissolved in. When dissolving
salt in water, the water is the solvent while the salt is the solute.
All living organisms obtain their food in one way or another through solutions.
Plants absorb nutrients through their roots only
when they are in solution.
We sometimes use the terms dilute,
concentrated and saturated
to describe the concentration of a solution in a non-exact way. The exact value of concentration
however, is what is really important to chemists and scientists. Concentration can be expressed in many different ways.
For example:
- grams of solute per litre of solution;
- mol of solute per litre of solution;
- mass of solute per mass of solution;
- volume of solute per volume of solution.
- parts per million (ppm)