Volumetric analysis
Indicators
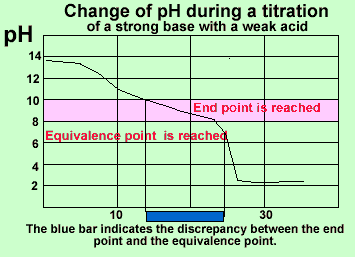
Phenolphthalein is not always a good choice as indicator. Look at the pH profile on the left. If phenolphthalein was used the end point would be reached long before the equivalence point. Almost 5 mL difference between the end point and the equivalence point would result. This would be unexceptable as an analysis procedure.
Some of the indicators and the pH range in which they change colour are listed below.
Phenolphthalein pH 8.2 - 10.0
Indigo carmine pH 11.6 - 14.0
Methyl violet pH 0.3 - 3.0
Methyl red pH 4.2 - 6.3
Methyl orange pH 3.1 - 4.5
In the analysis above it may
have been better to use methyl red instead of phenolphthalein. The colour
change would have occured soon after the equivalence point with minimal
addition of acid.
(In attempting these exercises it is assumed that the student is comfortable with stoichiometry and molarity)