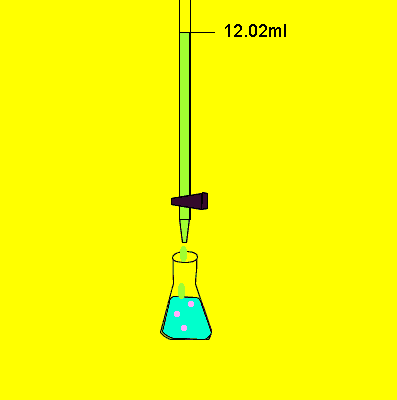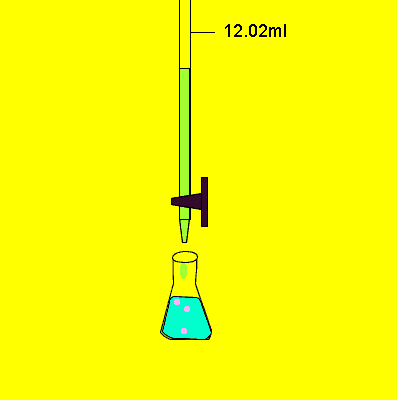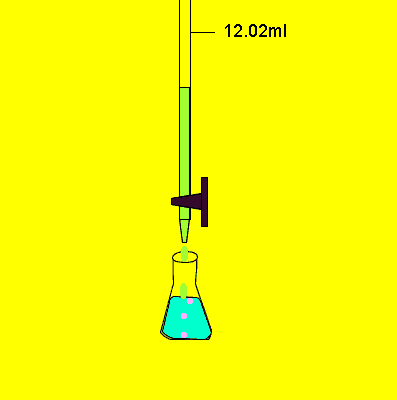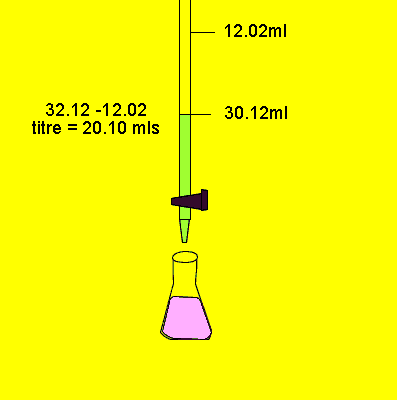
A typical back titration involves
the deliberate addition of excess reagent. Here we add the impure sample
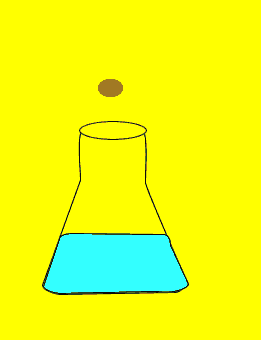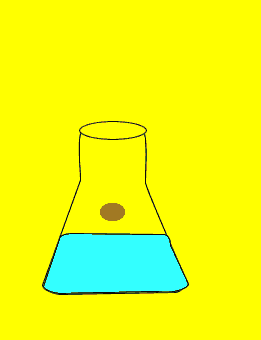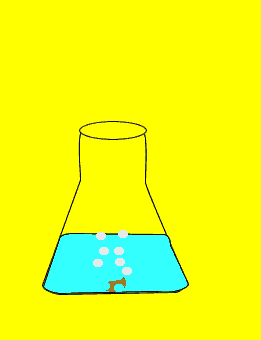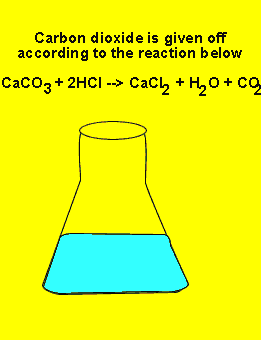
of limestone (calcium carbonate) to excess HCl. The reaction proceeds according
to the equation below. The excess amount of HCl can be determined very accurately.
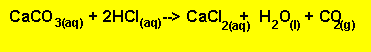
Next step is to now titrate
the solution with a base to determine how much acid remained unreacted.
This way we can work out exactly how much acid did react. We can then use
stoichiometry to determine the amount of calcium carbonate present.
Click
to hide this information
Solution
Step 1 - Work out the equations
for all the reactions taking place.
The equation for
the reaction betweenHCl with the limestone is given below.
Equation 1
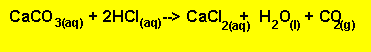
The equation for
the reaction between HCl and sodium carbonate is given below.
Equation 2
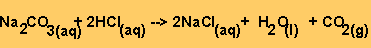
Step 2 - From equation
2 we can work out the mole of HCl left unreacted.
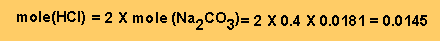
Step 3 - Work out
how much HCl did react. Subtract the amount in excess from the amount originally
added.
Amount originally
added - amount in excess = amount reacted.
(0.16 X 0.91) - 0.0145 = 0.1311 mole of HCl reacted.
Step 3 - Using equation
1 calculate the amount of caclium carbonate present.
Mole of HCl reacting /2 = mole of calcium carbonate = 0.1311/2 = 0.066 mole.
Step 4 - Calculate
the mass of calcium carbonate present.
0.066 X formula mass
= 0.066X 100 = 6.6 grams
Step 4 Calculate the
percentage
(mass of caclium carbonate
/ mass of sample) X 100 = %
6.6/8 X100 = 82.5%
Click
to hide the solution