Science of Conflict
Metallurgy-properties of metals.
Metals have very unique properties.
They are strong and usually melt at very high temperatures. Of course
the exception to this is mercury which is a liquid at room temperature.
Metals :
- are good conductors of electricity and
heat;
- can be polished and are very reflective;
- are malleable, that is, they can be beaten into different shapes;
- can be drawn into a wire, hence we call them ductile.
in order to understand why metals have such properties it is important
to see the atomic structure of a typical metal.
Ancient civilisations originally had uses for only a few properties of metals. The fact that metals are able to be polished and form shiny ornaments may have influenced their early uses. Gold is a soft, rare metal that is found in its pure form in the ground. Once found, it is relatively easy to process into jewelry as evident by its early use.
Ancient weapon makers looked
to metals for their hardness and malleability. Precious metals, such as
gold, are too soft to be useful as a structural materials or in the manufacture
of weapons. However, these shiny metals were highly valued.

This strong attraction in the metal structure is evident by the high temperatures necessary to melt metals.
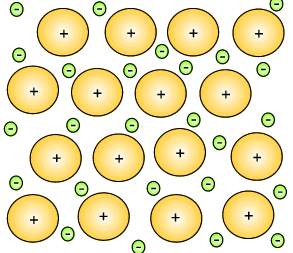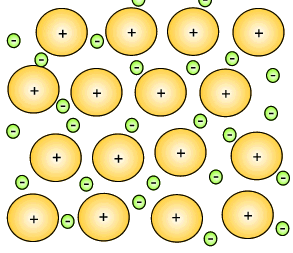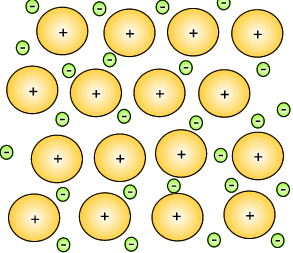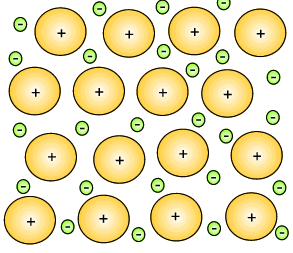
Mixtures of metals were combined to give metallic substances with totally different properties. A metal that consist of a mixture of different elements is known as an alloy. Iron can be hardened by mixing a small percentage of carbon in with the iron. This new substance, alloy, is known as steel. Copper can be hardened by mixing it with zinc to produce an alloy commonly known as brass. Copper mixed with tin creates bronze.