Volumetric analysis
Calculations
Volumetric analysis is a unique combination of calculations of molarity and stoichiometry.


|
Volume
|
|
| Start |
2.22 mls
|
| Finish |
22.22 mls
|
| Total |
20.00 mls
|
A commercial brand of vinegar was analysed for the its content of acetic acid, the active ingredient for vinegar. 20mls of the vinegar in question was pipetted straight from the bottle into a conical flask and two drops of indicator(pheolphthalein) added. Using a burette, 0.1M NaOH solution was slowly added until the indicator turned permanently pink. The volume of NaOH needed to turn the indicator pink is 20.00mls.
Calculate the concentration of acetic acid in the sample of vinegar.
The equation for the reaction is shown below.
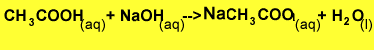
Step 1 Calculate
the mole of substance delivered from the burette. In this case NaOH.
Mole = Volume(litres) X Concentration
mole = 0.02 X 0.1 = 0.002 mole of NaOH
Step 2 Using
the equation calculate the number of mole of substance in the test sample.
In this case acetic acid.
According to the equation
above, one mole of NaOH reacts with one mole of acetic acid. So 0.002
mole of NaOH reacts with 0.002 mole of acetic acid.
Step 3 Find
the concentration of the substance in the test sample. In this case
find the concentration of acetic acid in 20 mls of vinegar.
Concentration = mole/Volume(litres) = 0.002/0.02 =0.1M
The acetic acid concentration in the vinegar sample is 0.1M